Transfusion medicine
Transfusion medicine handbook
The Transfusion Medicine Handbook is designed to assist hospital staff and other health professionals in modern Transfusion Medicine Practice.
5. Fractionated Products
5.1.2 PROTHROMBINEX-VF (Factors II, IX and X)
Prothrombinex-VF is a sterile freeze-dried powder containing purified human coagulation factors II, IX, and X. When reconstituted each vial contains 500 IU of factor IX, approximately 500 IU of factors II and X. The product formulated is with antithrombin III and heparin and contains small amounts of factors V and VII.
Prothrombinex-VF is prepared by adsorption of coagulation factors from plasma onto an ion-exchange medium followed by selective elution. The manufacturing process of Prothrombinex-VF contains dedicated steps including dry heat treatment and nanofiltration to reduce the potential for viral transmission. The current procedures are effective for inactivation/removal of HIV, hepatitis A, hepatitis B, and hepatitis C and may also have some effect on parvovirus B19.
The coagulation factors II, VII, IX and X, synthesised in the liver with the help of vitamin K, are together commonly called the prothrombin complex.
Prothrombinex-VF is a three-factor prothrombin complex concentrate (3FPCC). It is not interchangeable with Beriplex NZ, a four-factor prothrombin complex concentrate (4FPCC).
Acquired deficiency of the vitamin K-dependent coagulation factors occurs during treatment with coumarin vitamin K antagonists such as warfarin. It may also result from vitamin K deficiency due to malabsorption syndromes, antibiotic therapy, cholestasis or prolonged parenteral alimentation. If the deficiency progresses, a severe bleeding tendency results, characterised typically by retroperitoneal or cerebral bleeds rather than muscle and joint haemorrhage.
Severe hepatic insufficiency also results in markedly reduced levels of the vitamin K-dependent coagulation factors and may lead to a clinical bleeding tendency. The haemostatic situation is however often complex due to simultaneous ongoing low-grade intravascular coagulation, low platelet levels, deficiency of coagulation inhibitors and disturbed fibrinolysis.
Indications for Use
Prothrombinex-VF is indicated for:
- Treatment and perioperative prophylaxis of bleeding associated with acquired deficiency of prothrombin complex factors such as that caused by treatment with vitamin K antagonists, or in case of overdose of vitamin K antagonists, when rapid correction of the deficiency is required.
- Treatment and prophylaxis of bleeding associated with single (or multiple) congenital deficiency of factors IX, II or X when purified specific coagulation factor product is not available.
Section 6.10: Oral Anticoagulant – Warfarin Induced Bleeding or Overdose of this Handbook summarises the updated Australasian Society of Thrombosis and Haemostasis (ASTH) Consensus Guidelines for Warfarin Reversal published in the Medical Journal of Australia, 198 (4): 198-199, 2013. The recommendations for the use of prothrombin complex concentrate (PCC) in the setting of warfarin-related bleeding or warfarin overdose have been incorporated in the NZBS app Reversing Warfarin, available for iPhone and Android. Consultation with a specialist haematologist or NZBS Transfusion Medicine Specialist/Medical Officer is recommended.
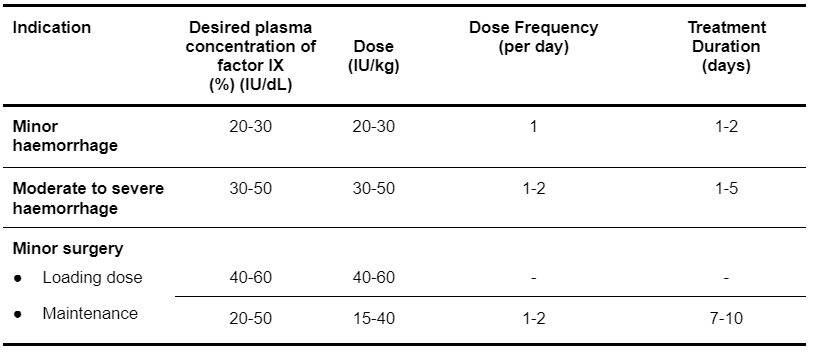
For congenital deficiencies of factors II, IX and X, the dosage and duration of the substitution therapy depend on the severity of the coagulation disorder, on the location and extent of the haemorrhage and on the clinical condition of the patient. The initial dose of a specific coagulation factor may be estimated from the recovery of that factor. In the absence of recovery data for Prothrombinex-VF, it is recommended that the following recovery data be used:
- 1 IU of factor II per kg body weight (IU/kg) raises the plasma factor II activity by 0.02 IU/mL.
- 1 IU/kg of factor IX raises the plasma factor IX activity by 0.01 IU/mL. General guidance on dosing is shown in Table 5.3.
- 1 IU/kg of factor X raises the plasma factor X activity by 0.017 IU/mL.
The calculation is as follows:
Dose (IU) = Body weight (kg) x Desired Factor Rise (IU/mL) x the reciprocal of the estimated recovery
Prothrombinex-VF should not be infused at a rate greater than 3 mL/minute.
Table 5.3: Prothrombinex-VF Dosing Guideline for Haemophilia B
Precautions
- Allergic reactions
Allergic reactions are rarely observed although severe anaphylaxis has been reported, particularly in patients with factor IX inhibitors. Depending on the nature of an adverse reaction, the rate of injection should be slowed or stopped to alleviate symptoms. - Antibodies to factor IX
Patients with congenital factor IX deficiency may develop neutralising alloantibodies (inhibitors) to factor IX after treatment. The reported prevalence for the formation of inhibitors in patients receiving plasma-derived factor IX is approximately 4%. - Antifibrinolytic agents
Use of Prothrombinex-VF with tranexamic acid is not recommended. Limited data is available on the concomitant administration of prothrombin complex concentrates and antifibrinolytic agents. - Heparin
Prothrombinex-VF contains 200 IU heparin in each vial. Heparin is known to cause thrombocytopenia and the possibility of heparin-induced thrombocytopenia (HIT) syndrome should be considered if thrombocytopenia, with or without thrombosis, develops during treatment. Consideration should be given to the clinical effect of heparin if high doses of Prothrombinex-VF are required. - Thrombosis and DIC
Patients receiving Prothrombinex-VF, especially at doses greater than 50 IU/kg of factor IX or following repeated doses, may be predisposed to venous and arterial thromboembolism, DIC or myocardial infarction. It should be used with caution in neonates, in who immature hepatic function may lead to delayed clearance of activated coagulation factors and an increased risk of thrombotic complications.
