Transfusion medicine
Transfusion medicine handbook
The Transfusion Medicine Handbook is designed to assist hospital staff and other health professionals in modern Transfusion Medicine Practice.
4. Blood Components
4.11 Plasma Components
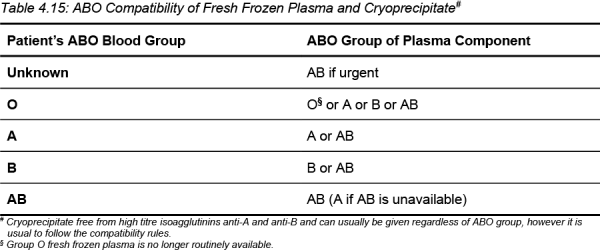
To avoid red cell haemolysis caused by transfusion of donor anti-A or anti-B, the ABO group of plasma components should be compatible with the ABO group of the recipient.
If high titre anti-A or anti-B are present in the plasma, the unit will be labelled accordingly and should only be transfused to a recipient with a compatible ABO group.
Table 4.15: ABO Compatibility of Fresh Frozen Plasma and Cryoprecipitate
RhD Compatibility
Although frozen plasma components may contain small amounts of red cell stroma, sensitisation following transfusion of RhD positive units is most unlikely, as stroma is less immunogenic than intact red cells. Therefore, FFP and cryoprecipitate of any RhD type may be given, regardless of the RhD type of the recipient. No prophylactic anti-D immunoglobulin need be given if RhD negative patients receive RhD positive FFP or cryoprecipitate.
Clinical Indications
Guidance on the indications for and dose of plasma components may be sought from a haematologist or NZBS Transfusion Medicine Specialist/ Medical Officer
Recommendations for the appropriate use of plasma components are available from Australasian and UK sources [7] [8] [9]
Adverse Reactions
Acute transfusion reactions to plasma components may be seen and include febrile non-haemolytic transfusion reactions (FNHTR), allergic reactions to plasma protein antigens, circulatory overload (TACO) following transfusion of volumes excessive for the patient, haemolytic reactions due to ABO incompatible plasma, bacterial contamination and transfusion-related acute lung injury (TRALI), although the latter has reduced in frequency following the introduction of male-only plasma (see Chapter 7: Adverse Effects of Transfusion).
Contraindications
It is not appropriate to use plasma components as a plasma expander or for replacement of plasma proteins in chronic hypoproteinaemic states. Fresh frozen plasma is generally not used for plasma exchange procedures except where performed for thrombotic thrombocytopenic purpura or in the presence of severe coagulopathy. The use of fresh frozen plasma is generally not considered appropriate for treating immunodeficiency states or for urgent reversal of vitamin K deficiency due to warfarin anticoagulation when a prothrombin complex concentrate (PCC) is readily available (see Table 4.16: Clinical Indications for Use of FFP).
