Transfusion medicine
Transfusion medicine handbook
The Transfusion Medicine Handbook is designed to assist hospital staff and other health professionals in modern Transfusion Medicine Practice.
5. Fractionated Products
5.1.4 RiaSTAP (Fibrinogen)
RiaSTAP is in limited supply so consultation with an NZBS Transfusion Medicine Specialist/ Medical Officer is required prior to release of this product.
RiaSTAP is a freeze-dried powder of purified human fibrinogen (factor I). Each 50 mL vial when reconstituted contains 1 g human fibrinogen for intravenous administration.
Indications for Use
RiaSTAP is indicated for:
- Prophylaxis and treatment of bleeding in patients with congenital fibrinogen deficiency including afibrinogenaemia and hypofibrinogenaemia.
Dosage and Administration
The (functional) fibrinogen level should be determined in order to calculate individual dosage requirements. The frequency of administration should be determined on an individual patient basis by regular measurement of plasma fibrinogen and continuous monitoring of the clinical condition of the patient. Dosage recommendations in the treatment of children are the same as for adults.
If the serum fibrinogen level is not known the recommended dose is 70 mg/kg body weight administered intravenously.
As a guide for subsequent dosing the target level of 1 g/L for minor events should be maintained for three days. The target level of 1.5 g/L for major events should be maintained for seven days.
Dose of fibrinogen = Target level (g/L) – Measured level (g/L)
(mg/kg body weight) 0.017 (g/L per mg/kg body weight)
Precautions
- Thrombosis
There is a risk of thrombosis when patients with congenital fibrinogen deficiency are treated with human fibrinogen, particularly with high or repeat doses. Patients, particularly those with risk factors for thromboembolic disease including neonates, should be monitored closely for signs and symptoms of thrombosis. - Allergic reactions
Serum sodium
RiaSTAP contains up to 164 mg (7.1 mmol) sodium per vial and this should be taken into consideration for patients on a sodium-restricted diet and/or with renal impairment.
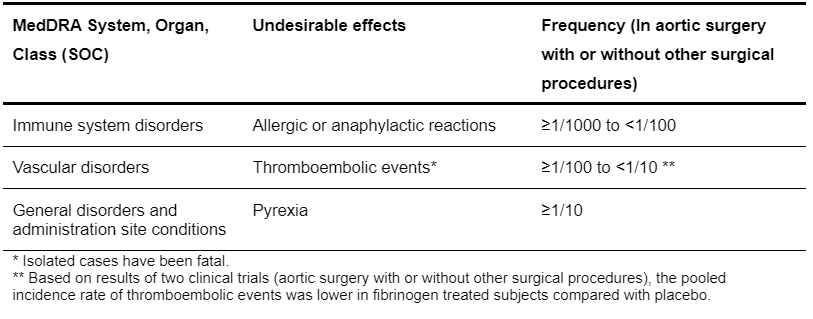
Adverse Events
Table 5.8: List of adverse drug reactions (ADRs) with RiaSTAP