Transfusion medicine
Transfusion medicine handbook
The Transfusion Medicine Handbook is designed to assist hospital staff and other health professionals in modern Transfusion Medicine Practice.
5. Fractionated Products
5.3.4 ALBUMEX 20 (Human albumin 20%)
Albumex 20 contains 200 g/L albumin in solution for intravenous injection and is hyper-oncotic and hypo-osmotic compared to human serum. It is available in vials of 10 mL and 100 mL volume. When infused it supplies the oncotic equivalent of approximately four times its volume of human plasma. Albumex 20 has two main functions: maintenance of plasma colloid osmotic pressure and transport of intermediate products in the transport and exchange of tissue metabolites. It is prepared by a combination of Cohn cold-ethanol fractionation and chromatography. The manufacturing process of Albumex 20 includes pasteurisation and cold temperature incubation to reduce the potential for viral transmission. The current procedures are effective for inactivation/removal of HIV, hepatitis A, hepatitis B, and hepatitis C and may also be of limited value against parvovirus B19.
Indications for Use
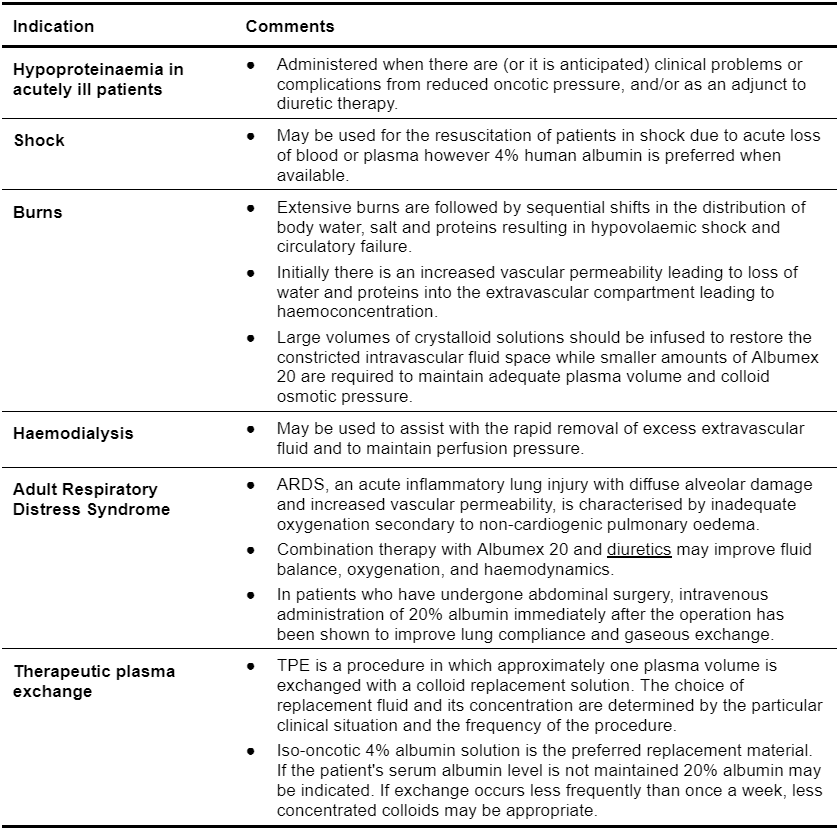
Table 5.8: Clinical Indications for Use of Albumex 20
Precautions
- Adverse effects
Adverse reactions to albumin solutions are uncommon and are usually mild and transient. Chills, fever, urticaria, flushing, nausea, headache and dyspnoea may occur. More serious allergic events including hypotension and anaphylaxis are reported. Hypotension has been reported in patients given albumin who are on angiotensin-converting enzyme (ACE) inhibitors. - Aluminium accumulation
Albumex 20 contains trace amounts of aluminium (≤ 200 µg/L). Accumulation of aluminium in patients with chronic renal insufficiency has led to toxic manifestations such as hypercalcaemia, vitamin D-refractory osteodystrophy, anaemia and severe progressive encephalopathy. Therefore, when large volumes of albumin are contemplated for administration to such patients, serious consideration should be given to these potential risks relative to the anticipated benefits. - Circulatory overload
The colloid osmotic effect of Albumex 20 is approximately four times that of plasma. Circulatory overload can be avoided by monitoring the rate and volume of infusion. Patients with cardiac failure, renal insufficiency or stabilised chronic anaemia often have an increased circulatory plasma volume and are at special risk of developing circulatory overload. - Compatibility with other fluids
Albumex 20 should not be mixed with protein hydrolysates, amino acid solutions, solutions containing alcohol, or solutions containing drugs that bind to albumin such as calcium channel blockers. - Hyperoncotic effects
As Albumex 20 is hyper-oncotic, it must be diluted with or followed by crystalloid solution in the presence of dehydration or shock.
If Albumex 20 is diluted to an iso-oncotic protein concentration (4-5% albumin) prior to administration, this must be done with an iso-osmotic electrolyte solution such as 0.9% saline. Under no circumstances should water be used since the lower tonicity will lead to intravascular haemolysis. - Hypoproteinaemia
The infusion of Albumex 20 is not justified in hypoproteinaemic states associated with chronic cirrhosis, malabsorption, protein losing enteropathies, pancreatic insufficiency, undernutrition. Albumex 20 may be indicated as a temporising measure in selected cases awaiting liver transplantation. - Nephrosis
In chronic nephrosis, infused albumin is promptly excreted by the kidneys with no or limited relief of the chronic oedema. Albumex 20 may be indicated as a temporising measure in selected cases awaiting renal transplantation. - Shock
Administration of albumin can aggravate myocardial depression when present in patients with shock. - Sodium levels
The sodium level in this product is 48 - 100 mmol/L. This should be noted when the product is used in patients requiring sodium restriction.
Administration
Albumex 20 supplied in a glass vial (bottle) and requires a vented infusion set to administer. The solution contains no antimicrobial preservatives; each bottle must be transfused within 4-hours of spiking (breaking the product seal with the IV set spike).
